PROTAC ER degraders are an investigational class of drugs designed to directly induce ER degradation.1-5
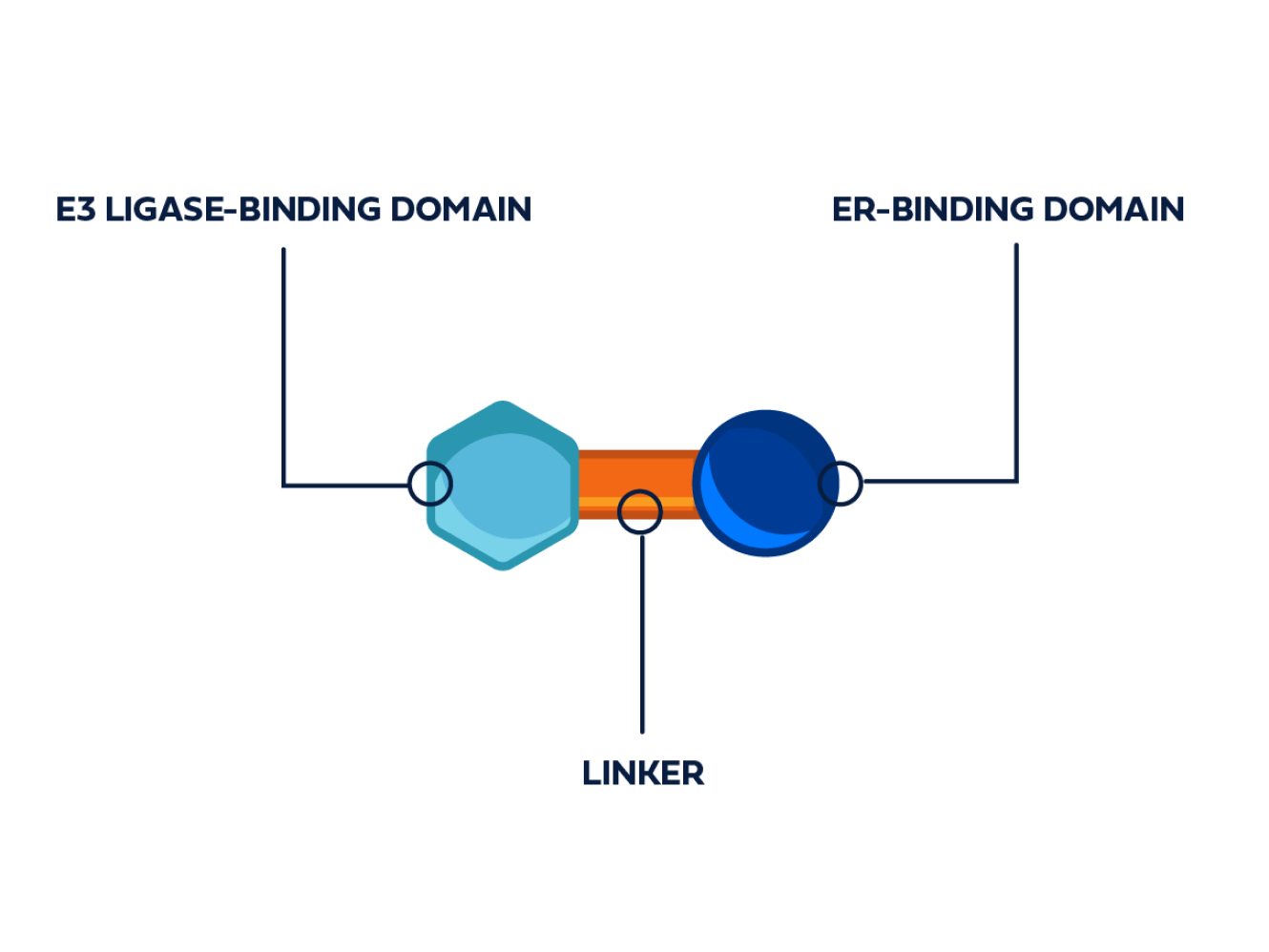
PROTAC ER degraders are bifunctional small molecules.2,6,7
In preclinical studies, PROTAC ER degraders were shown to degrade both wild-type and ESR1-mutated estrogen receptors in breast cancer cell lines.8-11
How PROTAC ER degraders are designed to work*:
Tap Swipe through the MOA to learn more
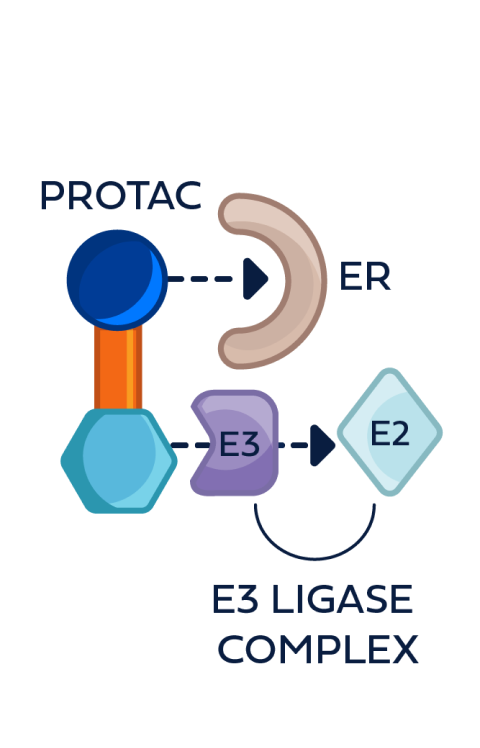
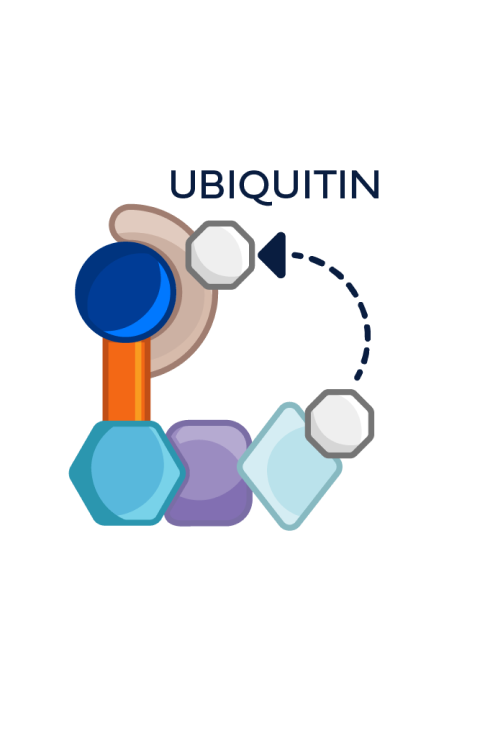
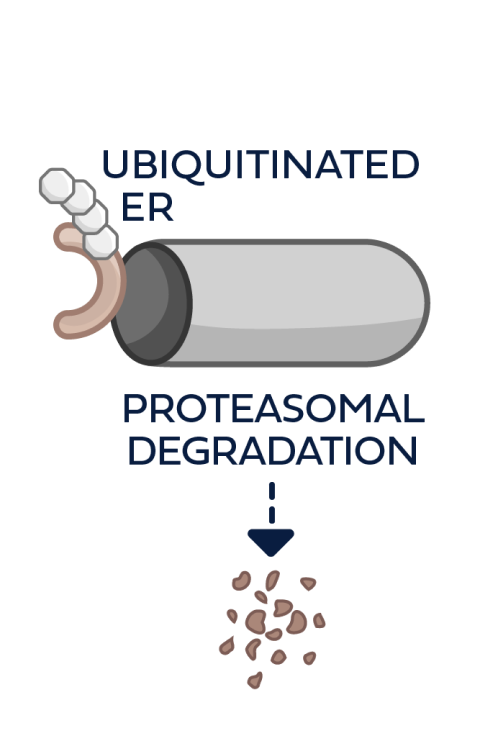
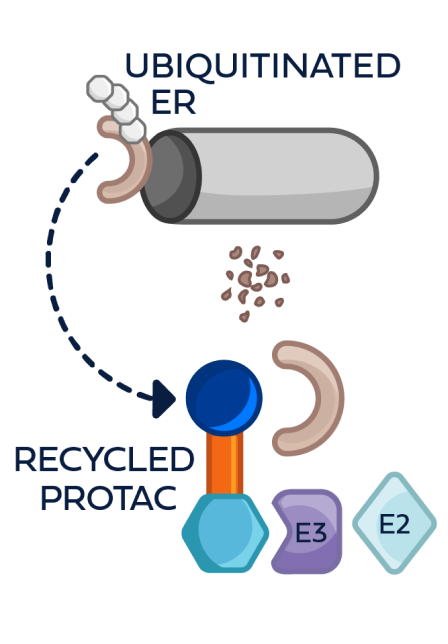
This process can be repeated multiple times, leading to the degradation of multiple ER molecules by a single PROTAC ER degrader until metabolized3,4,6
Continue to step 1

PROTAC ER degraders are an investigational class of drugs. Their safety and efficacy have not yet been established.
Mechanism of action for PROTAC ER degraders.
Watch Dr. Telli discuss how PROTAC ER degraders are designed to work.
Dr. Telli is a paid consultant of Arvinas.
PROTAC ER degraders are bifunctional small molecules composed of4,6:
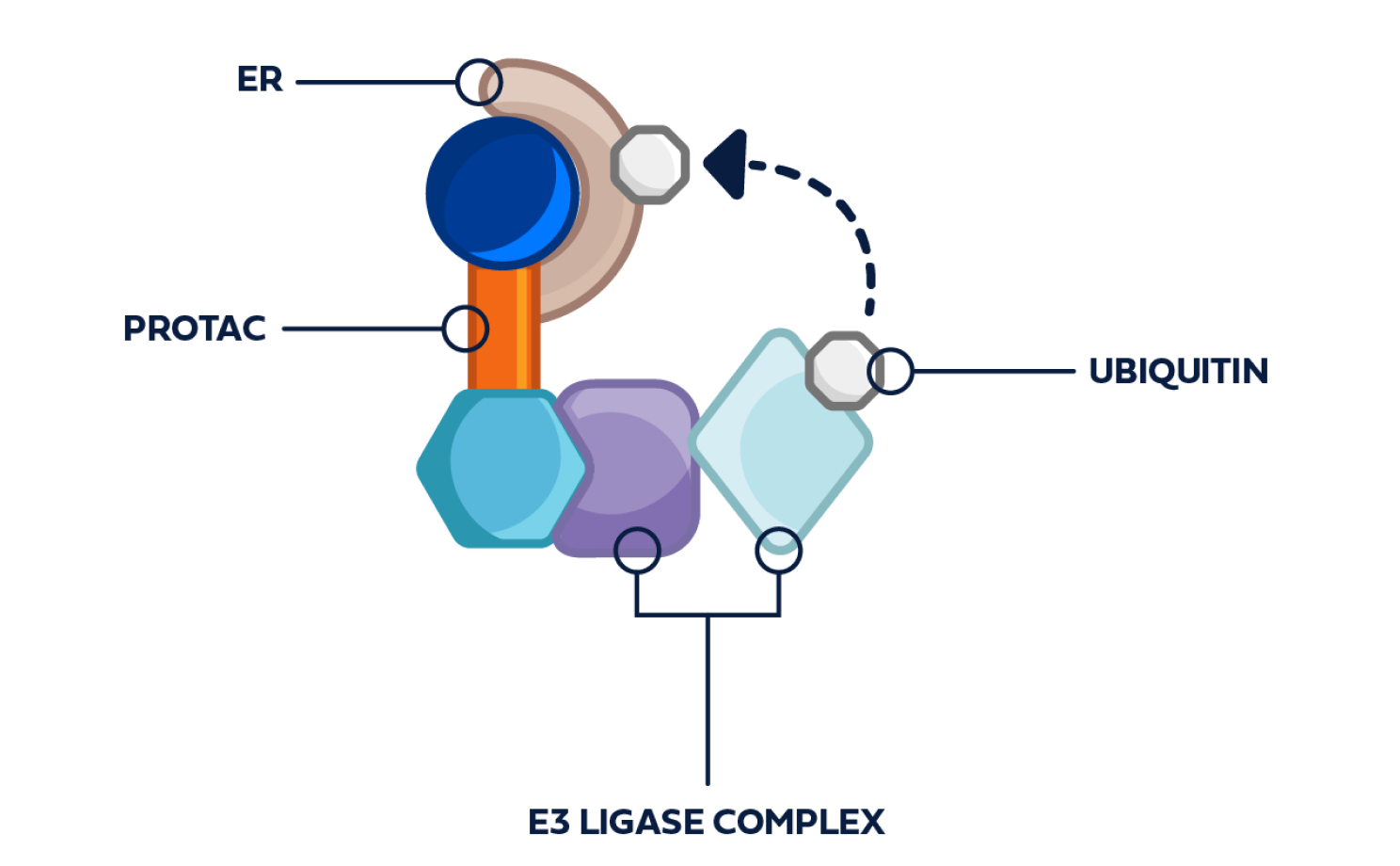
PROTAC ER degraders harness a natural cellular process to directly induce ER degradation.4,14
Proteins are degraded through the ubiquitin-proteasome system (UPS)4:
- The UPS functions as the body’s cellular protein disposal system
- Protein degradation by the UPS is part of normal, ongoing cellular maintenance that removes unneeded, misfolded, and/or damaged proteins
- E3 ligase is an important enzyme in the degradation process, as it facilitates the addition of the ubiquitin tag to the target protein
PROTAC ER degraders are being investigated in early and advanced ER+/HER2– breast cancer, alone and in combination with other treatment modalities.4,16-23
References: 1. Lloyd MR, Wander SA, Hamilton E, Razavi P, Bardia A. Next-generation selective estrogen receptor degraders and other novel endocrine therapies for management of metastatic hormone receptor-positive breast cancer: current and emerging role. Ther Adv Med Oncol. 2022;14:17588359221113694. doi:10.1177/17588359221113694 2. Patel R, Klein P, Tiersten A, Sparano JA. An emerging generation of endocrine therapies in breast cancer: a clinical perspective. NPJ Breast Cancer. 2023;9:20. doi:10.1038/s41523-023-00523-4 3. Liu Z, Hu M, Yang Y, et al. An overview of PROTACs: a promising drug discovery paradigm. Mol Biomed. 2022;3:46. doi:10.1186/s43556-022-00112-0 4. Békés M, Langley DR, Crews CM. PROTAC targeted protein degraders: the past is prologue. Nat Rev Drug Discov. 2022;21:181-200. doi:10.1038/s41573-021-00371-6 5. Hanker AB, Sudhan DR, Arteaga CL. Overcoming endocrine resistance in breast cancer. Cancer Cell. 2020;37:496-513. doi:10.1016/j.ccell.2020.03.009 6. Hernando C, Ortega-Morillo B, Tapia M, et al. Oral selective estrogen receptor degraders (SERDs) as a novel breast cancer therapy: present and future from a clinical perspective. Int J Mol Sci. 2021;22:7812. doi:10.3390/ijms22157812 7. Tecalco-Cruz AC, Zepeda-Cervantes J, Ramírez-Jarquín JO, Rojas-Ochoa A. Proteolysis-targeting chimeras and their implications in breast cancer. Explor Target Antitumor Ther. 2021;2:496-510. doi:10.37349/etat.2021.00060 8. Flanagan JJ, Qian Y, Gough SM, et al. ARV-471, an oral estrogen receptor PROTAC™ protein degrader for breast cancer. Presented at: San Antonio Breast Cancer Symposium; December 4-8, 2018; San Antonio, Texas. 9. He W, Zhang H, Perkins L, et al. Abstract PS18-09: Novel chimeric small molecule AC682 potently degrades estrogen receptor with oral anti-tumor efficacy superior to fulvestrant. Cancer Res. 2021;81(suppl 4):PS18-09. doi:10.1158/1538-7445.SABCS20-PS18-09 10. Rej RK, Chen Z, Acharyya RK, et al. Abstract 4510: Potent and orally efficacious PROTAC degraders of estrogen receptor α (ERα). Cancer Res. 2024;84(suppl 6):4510. doi:10.1158/1538-7445.AM2024-4510 11. Du W, Tu Z, Qin D, et al. Abstract LB179: Identification of the highly potent and orally available ER-targeting PROTAC degrader HP568 for the treatment of breast cancer. Cancer Res. 2024;84(suppl 7):LB179. doi:10.1158/1538-7445.AM2024-LB179 12. Ocaña A, Pandiella A. Proteolysis targeting chimeras (PROTACs) in cancer therapy. J Exp Clin Cancer Res. 2020;39:189. doi:10.1186/s13046-020-01672-1 13. Tecalco-Cruz AC, Ramírez-Jarquín JO, Macías-Silva M, Sosa-Garrocho M, López-Camarillo C. Novel breast cancer treatment by targeting estrogen receptor-alpha stability using proteolysis-targeting chimeras (PROTACs) technology. In: Mayrovitz HN, ed. Breast Cancer. Brisbane (AU): Exon Publications; August 6, 2022. 14. Lin X, Xiang H, Luo G. Targeting estrogen receptor α for degradation with PROTACs: a promising approach to overcome endocrine resistance. Eur J Med Chem. 2020;206:112689. doi:10.1016/j.ejmech.2020.112689 15. Sun X, Gao H, Yang Y, et al. PROTACs: great opportunities for academia and industry. Sig Transduct Target Ther. 2019;4:64. doi:10.1038/s41392-019-0101-6 16. A phase 1/2 trial of ARV-471 alone and in combination with palbociclib (IBRANCE®) in patients with ER+/HER2- locally advanced or metastatic breast cancer (mBC). ClinicalTrials.gov identifier: NCT04072952. Updated June 5, 2023. Accessed April 17, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT04072952 17. ARV-471 in combination with everolimus for the treatment of advanced or metastatic ER+, HER2- breast cancer (TACTIVE-E). ClinicalTrials.gov identifier: NCT05501769. Updated January 22, 2024. Accessed April 17, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05501769 18. TACTIVE-U: a study to learn about the study medicine (called ARV-471) when given with other medicines in people with advanced or metastatic breast cancer (sub-study A). ClinicalTrials.gov identifier: NCT05548127. Updated March 22, 2024. Accessed April 17, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05548127 19. A trial using ARV-471 or anastrozole in post-menopausal women with breast cancer prior to surgery. ClinicalTrials.gov identifier: NCT05549505. Updated January 22, 2024. Accessed April 17, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05549505 20. TACTIVE-U: a study to learn about the study medicine (called ARV-471) when given with other medicines in people with advanced or metastatic breast cancer (sub-study B). ClinicalTrials.gov identifier: NCT05573555. Updated April 5, 2024. Accessed April 17, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05573555 21. A study to learn about a new medicine called ARV-471 (PF-07850327) in people who have advanced metastatic breast cancer (VERITAC-2). ClinicalTrials.gov identifier: NCT05654623. Updated February 15, 2024. Accessed April 17, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05654623 22. A study of ARV-471 (PF-07850327) plus palbociclib versus letrozole plus palbociclib in participants with estrogen receptor positive, human epidermal growth factor negative advanced breast cancer (VERITAC-3). ClinicalTrials.gov identifier: NCT05909397. Updated April 15, 2024. Accessed April 17, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05909397 23. Study of AC699 in patients with estrogen receptor positive/human epidermal growth factor receptor 2 negative (ER+/HER2-) locally advanced or metastatic breast cancer. ClinicalTrials.gov identifier: NCT05654532. Updated February 7, 2024. Accessed June 3, 2024. https://classic.clinicaltrials.gov/ct2/show/NCT05654532
